Issue #82 – January 2022
NLPB Office Closed to the Public
As per current public health guidelines, the NLPB office is currently closed to the public and all staff members are working from home.
Email remains the best method for contacting NLPB. For staff contact information, please visit our website: https://nlpb.ca/contact-us/ or email general inquiries to inforx@nlpb.ca.
COVID-19 Pharmacy Service Disruptions
If your pharmacy has been affected by the recent increase in COVID-19 cases in the province, please refer to NLPB’s COVID-19 Guidance for Pharmacy Professionals webpage for guidance on navigating pharmacy service disruptions.
Please note that the pharmacist-in-charge should notify NLPB if/when they have pharmacy staff members that test positive for COVID-19 so we can be aware of the situation and any potential impacts on pharmacy services, such as a change in hours of operation or temporary closure. Patients should be notified of the changes to pharmacy services at the earliest opportunity, and continuity of care must be considered.
COVID-19 Testing and Isolation Guidelines for Health Professionals
As per the recent revision of the criteria for PCR testing and isolation, any person that is a close contact of a positive COVID-19 case and has symptoms of COVID-19 should consider themselves a positive case and follow public health direction. However, persons who work in health care can book a PCR test if they have been a close contact of a person who tested positive for COVID-19 regardless of whether or not they are exhibiting symptoms.
As per the public health guidance, a close contact is defined as any person exposed to the individual while they were infectious (72 hours before symptoms started). Exposed means anyone who:
- was within six feet of the individual for at least 15 minutes while indoors
- was nearby when they coughed or sneezed
- cares for you or you care for at home
- touched, hugged, or kissed you
Inter-provincial Prescription Transfer of Controlled Substances – Health Canada Subsection 56(1) Class Exemption
The Health Canada Subsection 56(1) Class Exemption for Pharmacists Prescribing and Providing Controlled Substances in Canada was updated in November 2021 to permit inter-provincial prescription transfers.
Pharmacists can now transfer a prescription for a controlled substance to another pharmacist within Canada for the duration of the Health Canada exemption. NLPB is seeking clarification from the provincial government regarding accepting out-of-province transfers as it pertains to the tamper-resistant prescription drug pad program (TRPP). In the meantime, all pharmacists should exercise their professional judgment when filling prescriptions.
Please note that only pharmacists who are authorized to prescribe by NLPB can continue prescriptions for controlled substances under the exemption.
For more information, check out NLPB’s updated FAQ:
https://nlpb.ca/media/NLPB-FAQ-on-Health-Canada-Exemption-Dec2021rev.pdf
Please reach out to the Department of Health and Community Services with any specific inquiries you may have.
Project ECHO NL: Opioid Use Disorder – Upcoming Sessions
Project ECHO NL: Opioid Use Disorder, is a virtual community advancing care and treatment for opioid use disorder in Newfoundland and Labrador. The goal is to help primary care providers and their teams build capacity in the treatment and management of opioid use disorder.
This project offers free, virtual learning sessions using an interactive, web-based platform to link healthcare providers with an interdisciplinary team of mentors with expertise in managing substance use care.
ECHO sessions are designed around case-based learning and mentorship and support healthcare providers in gaining the knowledge and skills required to provide needed services in their regions.
The primary audience for Project ECHO NL: Opioid Use Disorder is prescribers (family physicians and nurse practitioners) and pharmacists. The secondary audience is other healthcare professionals providing care for individuals with opioid use disorder, including registered nurses, licensed practical nurses, social workers, and addictions counsellors.
Upcoming Sessions
Cycle 1 will include 11 sessions held every three weeks between October 2021 and May 2022.
Session 4: Trauma-Informed Care in Addictions Practice
January 11, 2021 – 12:00–1:00PM (NST)
*This session was postponed due to the IT outage. If you are registered for the session when it was originally scheduled (Nov. 2, 2021), you do not need to register again.*
Registration is free but required. Registration is completed on a per-session basis.
Session 4 Registration Closed
ECHO sessions are one hour, held every three weeks on a rotating schedule of Tuesdays from 8:30 a.m. to 9:30 a.m. and 12:00 p.m. to 1:00 p.m. NDT.
| Session 3 | November 23, 2021 | 8:30 a.m.– 9:30 a.m. NDT |
| Session 4 | December 14, 2021 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 5 | January 11, 2022 | 8:30 a.m.– 9:30 a.m. NDT |
| Session 6 | February 1, 2022 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 7 | February 22, 2022 | 8:30 a.m.– 9:30 a.m. NDT |
| Session 8 | March 15, 2022 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 9 | April 5, 2022 | 8:30 a.m.– 9:30 a.m. NDT |
| Session 10 | April 26, 2022 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 11 | May 17, 2022 | 8:30 am – 9:30 am NDT |
More information about Project ECHO NL: Opioid Use Disorder, including frequently asked questions, can be found online here.
Issue #83 – February 2022
NLPB Office Closed to the Public
As per current public health guidelines, the NLPB office is currently closed to the public and all staff members are working from home.
Email remains the best method for contacting NLPB. For staff contact information, please visit our website: https://nlpb.ca/contact-us/ or email general inquiries to inforx@nlpb.ca.
Professional Development Audit Postponed
NLPB has decided to postpone the 2022 professional development audit due to the increased workload and stressors many pharmacy professionals are facing as a result of the ongoing COVID-19 pandemic. Traditionally, registrants are notified of their selection for the annual professional development audit by the end of January. NLPB will continue to monitor the situation and reassess this decision in the coming months. Registrants will be notified in advance should an audit proceed.
COVID-19 Pharmacy Service Disruptions
If your pharmacy has been affected by the recent increase in COVID-19 cases in the province, please refer to NLPB’s COVID-19 Guidance for Pharmacy Professionals webpage for guidance on navigating pharmacy service disruptions.
Please note that the pharmacist-in-charge should notify NLPB if/when they have pharmacy staff members that test positive for COVID-19 so we can be aware of the situation and any potential impacts on pharmacy services, such as a change in hours of operation or temporary closure. Patients should be notified of the changes to pharmacy services at the earliest opportunity, and continuity of care must be considered.
Tramadol Scheduling Changes Coming March 31, 2022
As of March 31, 2022, Health Canada will be amending the Prescription Drug List (PDL) to remove Tramadol. It will subsequently be listed in Schedule I of the Controlled Drugs and Substances Act (CDSA) and in the Schedule of the Narcotic Control Regulations (NCR), and therefore subject to all the regulatory requirements set out in the CDSA and NCR.
This change is intended to provide additional safeguards around the use of tramadol to help prevent problematic substance use and other harms, while also protecting access to these medications for patients who need them.
While Tramadol products sold and distributed by manufacturers after March 31, 2022, must include the updated labeling with the “N” narcotic symbol, in order to facilitate the transition and avoid disruption in market access, any products already sold and distributed by manufacturers prior to March 31, 2022, or product remaining at wholesalers or pharmacies, may continue to be sold until market depletion.
This change means that starting March 31, 2022, pharmacies will be expected to follow the same requirements for tramadol-containing products as they would for any other narcotic-containing product. This includes all requirements related to:
- Secure storage
- Recording of purchases and sales
- Perpetual inventory and physical inventory counts
- Filing and storage of prescriptions
- Documentation of destruction and/or returns
- Reporting of losses and/or thefts
- The use of the Health Canada “warning sticker” and patient information handout for opioids
For additional information, please see the related Controlled Substances Bulletin, section 1.6 of the Standards of Pharmacy Operation – Community Pharmacy and section 1.8 of the Standards of Pharmacy Operation – Hospital Pharmacy.
Please note that tramadol is already included in the list of drugs subject to the Tamper Resistant Prescription Pad Program.
Removal of Natural Health Products (NHPs) in Schedules I and II
In accordance with its Policy for Natural Health Products (NHPs), the National Association of Pharmacy Regulatory Authorities (NAPRA) has progressed with the planned removal of NHPs from the National Drug Schedules (NDS), in the stepwise, risk-based approach initiated in 2019.
Effective January 2, 2022, all NHPs that were listed within Schedules I and II were removed from the NDS, with the exception of ephedrine and pseudoephedrine. The removal of ephedrine and pseudoephedrine will occur on January 2, 2024. NHPs in the lowest risk categories, Schedule III and Unscheduled, were removed from the NDS in January 2020.
As of 2024, all products with a Natural Product Number (NPN) or Drug Identification Number-Homeopathic Medicine (DIN-HM) from Health Canada will be considered outside the scope of NAPRA’s NDS.
NAPRA believes the public interest will be best served through the development of a more comprehensive framework from the federal government that would better protect Canadians from the risks of the entire class of NHPs. As such, NAPRA will continue to engage in ongoing opportunities to further encourage the development of such a framework.
Paxlovid – Prescribing and Dispensing Information
Nirmatrelvir/Ritonavir (Paxlovid) is the first oral antiviral medication indicated for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death.
Due to the limited number of treatment doses, dispensing will initially be managed through the RHA pharmacies. A longer-term distribution plan is being developed which will include a role for community pharmacies when supply stabilizes. The initial supply of Nirmatrelvir/Ritonavir will not be available through standard pharmacy wholesale channels.
Individuals contacting community pharmacies regarding Paxlovid should be advised it is not yet available in community pharmacies and referred to their prescriber.
Prescribers are asked to complete the screening tool (see Resources below) and send it to the appropriate RHA pharmacy for processing and dispensing. Alternatively, if completion of the screening tool is not possible, a prescriber may telephone an identified site to verbally request treatment and provide the required screening information. The pharmacist will complete the screening tool on behalf of the physician and indicate that the prescription was received verbally. Screening tools completed in this manner are to be retained at the pharmacy for audit purposes. During dispensing, the RHA pharmacy will coordinate with the patient for delivery or contactless pickup.
More information will be released by the Department of Health and Community Services in the coming days as the treatment access evolves.
Resources
Nirmatrelvir/Ritonavir (Paxlovid) Guidance for Healthcare Professionals
Nirmatrelvir/Ritonavir (Paxlovid) Screening and Prescribing Form
Patient Connect NL
The Department of Health and Community Services, in partnership with Eastern Health, has launched a new initiative to develop a registry for all members of the public in the eastern region that are currently without a primary care provider. Members of the public can self-register with Patient Connect NL and be waitlisted for a primary care provider at the new Collaborative Team Clinics.
Pharmacy professionals in the eastern region who become aware of patients who do not have a family doctor are encouraged to refer them to the program.
The program is in development to be expanded provincially but is currently only operational for the eastern region.
Pharmacies located in the eastern region can print and display the resources below:
Resources
Patient Connect NL Poster
Patient Connect NL Business Card
Update – Reporting Loss or Theft of Controlled Substances or Precursors
Pharmacists can now self-register to Health Canada’s E-Services Portal to report loss or theft of controlled substances or precursors and no longer need to be invited onto the system.
A call centre is also available to provide technical support: csps-spsc@hc-sc.gc.ca or 1-866-337-7705 (option 5)
Resources
Path of Portal Reporting
Why Use the Portal
Guidance on reporting loss or theft of controlled substances and precursors
For more information, visit Health Canada’s website: https://www.canada.ca/en/health-canada/services/health-concerns/controlled-substances-precursor-chemicals/controlled-substances/compliance-monitoring/loss-theft.html
Invitation to Participate in Opioid Dependence Treatment Needs Assessment
The Provincial Opioid Dependence Treatment Centre of Excellence is inviting pharmacy team members to participate in an Opioid Dependence Treatment needs assessment to determine how the Centre of Excellence can better support pharmacy teams in their practices, with the treatment of their patients, and increase knowledge surrounding opioid dependence treatment and harm reduction. The content was developed in close consultation with Sydney Peckham, Clinical Lead (Pharmacy) with the Centre of Excellence and regional ODT hubs, and PharmD students completing non-clinical placements with the COE.
This needs assessment targets pharmacists, pharmacy technicians, pharmacy assistants, and pharmacy students. It is designed to be completed individually, as each group has a set of questions that is applicable to their role.
What is the Provincial Opioid Dependence Treatment Centre of Excellence?
The Provincial Opioid Dependence Treatment (ODT) Centre of Excellence (COE) was created as part of the Hub and Spoke model for ODT across the province. While located in Eastern Health, the COE supports all health authorities and regions of Newfoundland and Labrador to help build capacity around ODT services. Goals of the COE include:
- Supporting the implementation of the provincial ODT Hub and Spoke model
- Supporting the implementation of evidence-informed ODT practices
- Strengthening ODT performance monitoring and evaluation
- Increasing opportunities for stakeholder collaboration
- Strengthening harm reduction education, program, policy, and practice development
- Increasing opportunities for knowledge exchange and care provider development
Privacy Details and Contact Information:
This needs assessment will be administered in a way that protects your privacy. It is your choice whether or not you complete the needs assessment; if you choose not to participate, it will not affect your employment in any way. If you choose to fill out the needs assessment, please answer as best you can. It should take about 10 – 15 minutes to complete.
Needs Assessment: https://www.surveymonkey.com/r/Needs_Assessment_for_Pharmacy_Teams_2022.
Please complete the needs assessment by March 1, 2022.
If you have any questions or if you would like to have this needs assessment in another format, please contact Kate Lambert, Knowledge Exchange Facilitator, at kate.lambert@easternhealth.ca or 752-3573.
Project ECHO NL: Opioid Use Disorder – Upcoming Sessions
Project ECHO NL: Opioid Use Disorder, is a virtual community advancing care and treatment for opioid use disorder in Newfoundland and Labrador. The goal is to help primary care providers and their teams build capacity in the treatment and management of opioid use disorder.
This project offers free, virtual learning sessions using an interactive, web-based platform to link healthcare providers with an interdisciplinary team of mentors with expertise in managing substance use care.
ECHO sessions are designed around case-based learning and mentorship and support healthcare providers in gaining the knowledge and skills required to provide needed services in their regions.
The primary audience for Project ECHO NL: Opioid Use Disorder is prescribers (family physicians and nurse practitioners) and pharmacists. The secondary audience is other healthcare professionals providing care for individuals with opioid use disorder, including registered nurses, licensed practical nurses, social workers, and addictions counsellors.
Upcoming Sessions
Cycle 1 will include 11 sessions held every three weeks between October 2021 and May 2022.
Session 6: Microdosing as an Induction Approach
February 22, 2022 – 8:30–9:30AM (NST)
Registration is free but required. Registration is completed on a per-session basis.
Register Today The deadline to register is February 18, 2022
ECHO sessions are one hour, held every three weeks on a rotating schedule of Tuesdays from 8:30 a.m. to 9:30 a.m. and 12:00 p.m. to 1:00 p.m. NDT.
| Session 7 | March 15, 2022 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 8 | April 5, 2022 | 8:30 a.m.– 9:30 a.m. NDT |
| Session 9 | April 26, 2022 | 12:00 p.m.– 1:00 p.m. NDT |
| Session 10 | May 17, 2022 | 8:30 am – 9:30 am NDT |
More information about Project ECHO NL: Opioid Use Disorder, including frequently asked questions, can be found online here.
Issue #84 – May 2022
New NLPB Registrant Portal Coming Soon!
In order to better support registrants, NLPB is moving to a new registrant portal. To allow for the transfer of information, including all registrant profiles and learning portfolio records (CEs), as of Monday, May 2, 2022, the registrant portal is no longer accessible. NLPB is expecting the launch of the new registrant portal by the end of June. We apologize for any inconvenience this may cause. We look forward to introducing our enhanced portal to you in the near future.
2022 Annual General Meeting
NLPB’s Annual General Meeting will be taking place 10:00am-11:00am on Friday, May 13, 2022 via Zoom.
Zoom Meeting Link: https://us02web.zoom.us/j/83449928441?pwd=d25hOFhwVUhJMWtub0pBT1JaVUIvQT09
Meeting ID: 834 4992 8441
Passcode: 466433
Agenda:
1. Call to Order and Adoption of Agenda
2. Introduction of Board Members
3. Minute of Silence in Memoriam
4. Highlights of 2021 Annual Report
5. Questions on Annual Report
6. Appointment of Auditor for 2023
7. Board Member Election Results
8. Adjournment
While our condensed AGM will not include an open forum for registrants, the NLPB 2021 Annual Report will be published on the NLPB website at: https://nlpb.ca/about-the-board/. If you have questions about the report, please contact us at inforx@nlpb.ca.
Now Hiring – Office Administrator
NLPB is now hiring for the full-time position of Office Administrator. For full details please visit the NLPB website: https://nlpb.ca/now-hiring-office-administrator/
Qualified applicants are invited to submit a resume and a cover letter outlining why they are interested in this opportunity and how their work experience has prepared them for this role. Submissions without a cover letter will not be considered. Email your resume and cover letter to careers@nlpb.ca. The application deadline for this position is May 27, 2022.
Health Canada – Submission of Loss or Theft Reports – Fax Decommissioned
Registrants are advised that the Health Canada’s Office of Controlled Substances fax line for controlled substance and precursor Loss or Theft Reports has been decommissioned due to technical issues.
If you have attempted to submit a report by fax since April 5, 2022, and it did not go through, please resubmit using one of the methods identified below:
Please Note: Pharmacists can now self-register to Health Canada’s E-Services Portal to report loss or theft of controlled substances or precursors and no longer need to be invited onto the system. A call centre is also available to provide technical support: csps-spsc@hc-sc.gc.ca or 1-866-337-7705 (option 5)
For more information, please visit the Health Canada Website or contact them at ocs.reporting-rapporter.bsc@hc-sc.gc.ca.
Tips for Application Submission
NLPB receives many applications for processing. Unfortunately, some of the applications submitted are illegible, incomplete, or missing supporting documentation or payment, leading to delays in processing and approving the application. In order to ensure that your application is processed in a timely manner, please take a moment before submission to ensure that all fields are completed and legible and that all supporting documentation is included. These small steps will help improve the process for both the applicant and NLPB.
Anti-Stigma Training Materials
Stigma and discrimination pose significant barriers that prevent people from seeking support, treatment, and harm reduction services in their communities. There are many ways to assist with increasing understanding and reducing stigma. One way is to consider the language we use as a powerful tool to counter stigmatizing attitudes and behaviors. When we reframe mental illness and substance use disorders as treatable medical conditions from which recovery is possible, we reduce blaming and correct the misconception that these conditions occur because of people’s choices. We all have a part to play in eliminating stigma and discrimination.
Anti-Stigma and Discrimination Resources:
- Overcoming Stigma Through Language provides free online learning modules developed in partnership with Community Addictions Peer Support Association (CAPSA). https://www.ccsa.ca/overcoming-stigma-online-learning
- Language Matters is a quick reference guide to help combat stigma by changing the language that we use to discuss people with mental illness and substance use disorders. https://mhfa.ca/en/safer-language-reference-guide
- Understanding Stigma – The Mental Health Commission of Canada (MHCC) provides this free self-directed course is available in both official languages and consists of three modules that focus on raising awareness, the impacts of stigma, and challenging stigma and discrimination. www.understandingstigma.ca
- Beyond Stigma – The following short animated video highlights the stigma people with Substance Use Disorder face when seeking primary health care and how it can be overcome. It too was created from a multidisciplinary group with pharmacists, clinicians, and persons with lived experience. Beyond Stigma on Vimeo
- Understanding Changes Everything – this three-minute video was produced in Newfoundland and Labrador with people with lived experience with mental illness. It depicts the impact of misconceptions about mental illness. Understanding Changes Everything
- Brain Story in Action: Addressing Stigma – another short video about how brain science is helping to address the stigma of addiction in Newfoundland and Labrador. Brain Story in Action: Addressing Stigma – YouTube
- A Resource for Canadian Health Professional Organizations, developed with people with lived and living experience of substance and their loved ones, was designed to facilitate reflections on the use of language on substance use topics. Language sets a tone for people and can help reduce both public and systemic substance use stigma. https://www.canada.ca/en/public-health/services/publications/healthy-living/communicating-about-substance-use-compassionate-safe-non-stigmatizing-ways-2019.html
Issue #85 – June 2022
NLPB Registrar Announces Intention to Retire
Registrar, Margot Priddle, has announced her intention to retire from her position with NLPB in the fall of 2022. Margot has served as Registrar of NLPB for the last decade, with many noted advancements to the pharmacy profession and its regulation taking place under her leadership. Please read the full retirement announcement, available on the NLPB website for more details regarding Margot’s tenure as Registrar.
NLPB has contracted Knightsbridge Robertson Surrette to begin the process of recruiting a new Registrar.
New NLPB Registrant Portal Coming Soon!
In order to better support registrants, NLPB is moving to a new registrant portal. As of Monday, May 2, 2022, the registrant portal was no longer accessible in order to allow for the transfer of information, including all registrant profiles and learning portfolio records (CEs). NLPB is expecting the launch of the new registrant portal by the end of June. We apologize for any inconvenience this may cause. We look forward to introducing our enhanced portal to you in the near future.
NLPB’s Public Register
The Public Register is currently available by download only.
As NLPB moves to a new registrant portal, the current registrant portal from which the Public Register is generated is currently not accessible. As such, the register will be updated manually as registrant or licensee information changes. The date of the last update can be viewed above the links to each section of the register on the website. Should you have any questions or need further clarification regarding NLPB registrants and/or licensed pharmacies, please contact us at inforx@nlpb.ca.
The NLPB Team
SPOTLIGHT ON QUALITY ASSURANCE
NLPB’s Quality Assurance (QA) team is responsible for administering the QA program. The QA team aims to advance continuous quality improvement processes so that the program can have the greatest impact on the quality and safety of pharmacy practice.
NOELLE PATTEN (SHE/HER)
Noelle is the Associate Registrar at NLPB who oversees the Quality Assurance and Licencing portfolios.
Over the past 8 years, she has visited most community and hospital pharmacies in the province to assist them with meeting NLPB standards for pharmacy operation and practice. She is also responsible for ensuring pharmacy professionals meet their yearly professional development requirements and practice expectations, which includes coordinating the activities of the Professional Development Review Committee and Quality Assurance Committee.
Noelle graduated from Memorial University’s School of Pharmacy in 2006. For the first 8 years of her career, she enjoyed practicing in community pharmacy, with a focus on management of Opioid Use Disorder. Joining NLPB further sparked her interest in health policy, leading her to complete a graduate degree in Health Management from McMaster University and a Certified Health Executive designation from the Canadian College of Health Leaders. Noelle is passionate about optimizing pharmacy professionals’ roles within the health system to improve access to healthcare and health outcomes. She looks forward to continuing to learn about effective regulation and quality improvement strategies.
KEN WALSH (HE/HIM)
Ken is a practice consultant and pharmacy practice site assessor for hospital pharmacies with NLPB’s QA Team.
In this position, he conducts assessments of hospital pharmacies and addresses practice questions from hospital pharmacy professionals throughout the province. Over the past 3 years, he has been leading hospital pharmacy teams through the implementation of new standards for sterile and non-sterile compounding, which represent a significant change to compounding practice and pharmacy infrastructure. To prepare him for this key role, Ken received sterile compounding inspector training at Critical Point in the United States and maintains this skillset through continuing education. He also represents NLPB on NAPRA’s sterile compounding standards working group.
Ken is a graduate of the College of Trades and Technology Pharmacy Program and has a Bachelor of Technology (Health Science) degree from Memorial University. He is well-positioned to mentor hospital pharmacy teams, as he worked as a hospital pharmacist for 34 years, the last 25 years of which he was the Regional Director of Pharmacy for Western Health. Ken looks forward to bringing hospital pharmacy teams to the “finish line” in meeting compounding standards and continuing to grow the hospital pharmacy practice site assessment program.
RHEYA WHITE (THEY/THEM)
Rheya is a practice consultant and practice site assessor for community pharmacies as part of the QA team.
Presently Rheya works with pharmacy professionals across the province, providing guidance on questions related to pharmacy practice and operational standards. Additionally, they facilitate practice site assessments for community pharmacies, working directly with pharmacists-in-charge to promote quality assurance and continuous quality improvement within a community pharmacy setting.
Rheya entered the profession in 2008 upon graduating from Memorial University’s School of Pharmacy. Since that time, they have had the opportunity to work with a variety of community pharmacy teams as a pharmacist, pharmacy district manager, and now as a member of NLPB’s QA team. Rheya looks forward to their continued work with others in hopes of ensuring safe, progressive and equitable care for all.
2021 Annual Report
NLPB’s 2021 Annual Report is now available for viewing online at https://nlpb.ca/media/Annual-Report-2021.pdf
Issue #86 – September 2022
New NLPB Registrant Portal Set to Launch This Month!
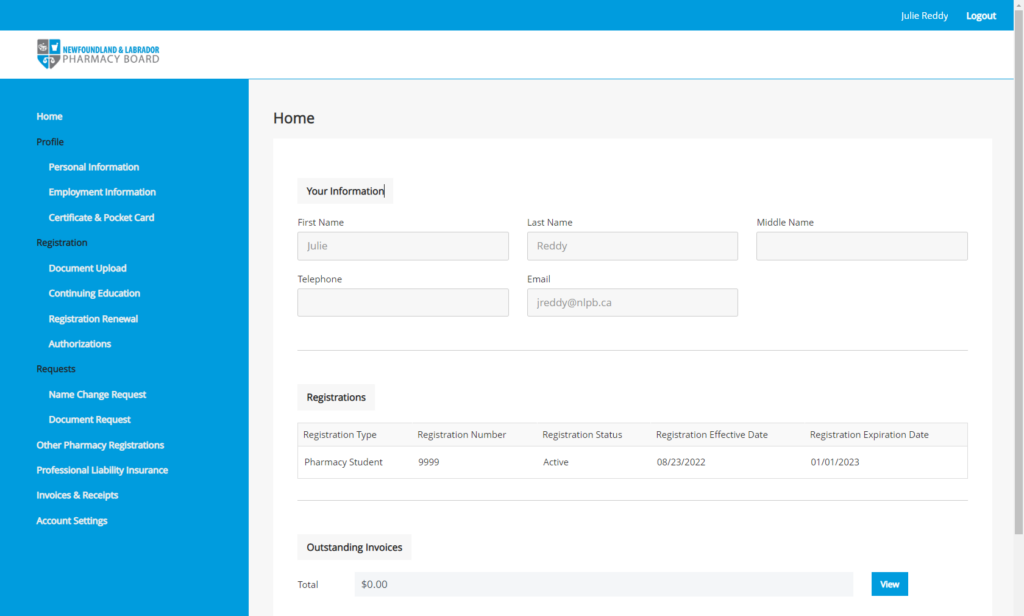
NLPB’s new Registrant Portal, powered by Thentia Cloud, will launch to all registrants in September. So, keep an eye on your inboxes in the coming weeks for an email from nlpb.registration@thentiacloud.com with instructions for logging into your profile.
Once you are given access to your profile, it is recommended that you review and update your information as necessary. You can navigate your profile by using the menu on the left side of the screen. Key sections you may wish to review, include Personal Information, Employment Information, Continuing Education, Authorizations, and Professional Liability Insurance. If your Professional Liability Insurance has expired or was renewed while the Registrant Portal was inaccessible, please update the information and upload a current policy certificate.
In preparation for registration renewal, you can record your completed continuing education units in the registrant portal. For those who have been recording their activities in the Learning Activities Notes form, you can copy and paste the information in the digital form to the corresponding fields in the registrant portal.
Should you have any questions or concerns regarding navigating the portal or updating your profile information, please contact NLPB at registration@nlpb.ca for assistance.
| Following a standard Request for Proposals, NLPB made the decision to move to a new registrant management system in the first quarter of this year. The decision was made to better support registrants as well as enhance the staff’s ability to manage key business functions for which NLPB is responsible. To make this transition, access to the registrant portal has been restricted for a period. NLPB would like to thank registrants for their patience throughout this process.
During this transition period, NLPB staff have been maintaining and updating all registrant profiles, while our platform developers have been configuring the system to ensure registrants will have access to everything they need to manage their profiles and renew their registration on time. |
The Registrant Portal is just one of several portals that are in development as part of NLPB’s new registrant management system. The Pharmacy Portal, which will allow pharmacists-in-charge to manage their pharmacy profile and renewals is on schedule to launch in October, prior to the opening of license renewals. The Public Register is also on schedule to be fully functional at that time.
Once these scheduled launches are complete, NLPB will be working on moving our applications and complaints & discipline processes online.
NLPB’s Public Register
The Public Register is currently available by download only.
As NLPB moves to a new registrant portal, the current registrant portal from which the Public Register is generated is currently not accessible. As such, the register will be updated manually as registrant or licensee information changes. The date of the last update can be viewed above the links to each section of the register on the website. Should you have any questions or need further clarification regarding NLPB registrants and/or licensed pharmacies, please contact us at inforx@nlpb.ca.
Model Standards of Practice for Pharmacists and Pharmacy Technicians
The National Association of Pharmacy Regulatory Authorities’ (NAPRA) new Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada was adopted by the NLPB board of directors on August 8, 2022, during a regular meeting of the board. These model standards were approved by the NAPRA Board in the fall of 2021 and published on the NAPRA website in February 2022. A link to the standards is now available on the NLPB website.
National Drug Schedule Updates – Topical Diclofenac Diethylamine
National Drug Scheduling Advisory Committee (NDSAC) finalized the following recommendations, effective July 26, 2022:
- Diclofenac diethylamine – for human use – when sold as a single medicinal ingredient for topical use on the skin in concentrations greater than 1.16% and less than or equal to 2.32% for not more than 7 days – in package sizes containing greater than 2.6g of diclofenac diethylamine be granted Schedule III status
- Diclofenac diethylamine – for human use – when sold as a single medicinal ingredient for topical use on the skin in concentrations greater than 1.16% and less than or equal to 2.32% for not more than 7 days – in package sizes containing no more than 2.6g of diclofenac diethylamine be granted Unscheduled status
- Diclofenac diethylamine – for human use – when sold as a single medicinal ingredient for topical use on the skin in concentrations of not more than 1.16% for not more than 7 days remain Unscheduled
For more information or to subscribe to National Drug Schedule Updates, visit the NAPRA website.
Risk Management Plans for Health Care Professionals
Health Canada’s Marketed Health Products Directorate released a new Q&A document in July 2022 to provide clarification on frequently asked questions regarding Risk Management Programs, including Controlled Distribution Programs. This document will be temporarily available on NLPB’s website for review and download: Questions & Answers: Role of Health Canada and the Risk Management Plan in the Product Lifecycle.
Prescription Monitoring Program – NL
The Prescription Monitoring Program – NL helps ensure that you, as pharmacy professionals, are making the most informed decisions when choosing to prescribe or dispense a monitored drug. Stay up to date on the program by reviewing their quarterly Newsletters.
Issue #87 – October 2022
Annual Registration and Licence Renewals
Registration Renewals
NLPB’s annual renewal period for registrants opens today, October 24, 2022. As this will be the first renewal period using the new NLPB Registrant Portal, please refer to the NLPB Registrant Portal User Guide – Renewing Registration for step-by-step instructions for successfully renewing through the online system.
As the online registrant portal was inaccessible for several months this year during the transition to the new system, NLPB will be extending the renewal period for pharmacists and pharmacy technicians by two additional weeks to allow registrants time to enter their required professional development activities prior to renewal. The deadline to renew your registration is Thursday, December 15, 2022. All continuing education units (CEUs) must be entered and all fees paid by that date.
Licence Renewals
Due to a technical bug in the Thentia Cloud system, both the launch of the Pharmacy Portal and the opening of the Pharmacy Licence Renewals have been delayed. The renewal period for pharmacies is now scheduled to begin on November 15, 2022. Pharmacists-in-charge will receive an email notification at the opening of renewals, which will include a complete guide to renewing the pharmacy licence through the online system. Please note, the pharmacist-in-charge must first renew their registration before renewing the pharmacy licence.
Before renewing, pharmacists-in-charge should ensure they have a current list of all owners (including profession, mailing address, phone number, and email address) and a list of all registered and non-registered staff members (including pharmacy assistants and clerks).
The deadline to renew the pharmacy licence is Thursday, December 15, 2022.
The pharmacist-in-charge is responsible for ensuring the licence has been renewed for 2023 and all regulated staff is registered to practice in 2023 before the end of the year.
Please note: Registration is not complete until all fees are paid. Please refer to the 2023 Schedule of Fees for a list of applicable fees.
Logging in to the New Registrant Portal
All registrants should have received an email containing their login information and instructions for accessing the registrant portal on Thursday, September 22, 2022. If you are currently an NLPB registrant and have not yet received your login information for the new registrant portal, please first check your Junk Mail folder before contacting registration@nlpb.ca for assistance.
To access the portal please visit: https://nlpb.portalca.thentiacloud.net/webs/portal/service/#/login
Registrants are reminded that they must use the email address identified in the login information email to access the system. If logging into the system for the first time, click the Forgot Password button and enter your email address to have a new temporary password sent to you. Follow the instructions in the email to access the system.
Once logged into the NLPB Registrant Portal, registrants will be asked to create a new password and security questions. Please note that both passwords and security questions are case-sensitive.
When logged into the NLPB Registrant Portal, please be advised that, for security purposes, the system will automatically log you out of the portal after 15 minutes of inactivity. The system does not recognize when a registrant is typing in a field, so if more than 15 minutes are spent typing in a single field, the information will not be successfully saved.
Recording Professional Development Activities in the Registrant Portal
All registrants are required to complete 15 CEUs between December 1, 2021 and November 30, 2022 to be eligible to renew their annual registration. A minimum of 7.5 CEUs must be accredited and the remaining may be either accredited or self-assigned.
To record your completed professional development activities in the registrant portal, follow the steps outlined in the NLPB Registrant Portal User Guide – Adding Professional Development Activities.
To assist registrants in adjusting to the 15-minute inactivity session timeout security feature, below are some tips for recording and successfully submitting learning activities.
- Save supporting documents in a dedicated location on your device so they are easy to find when you are ready to upload the files.
- Have your notes on hand when you are recording learning activities in the registrant portal.
- Make notes in a document on your computer so you can copy and paste the information into the online form in the registrant portal.
- When creating a new record, first upload the supporting document, enter the Program Title, insert placeholder text (i.e. TBD), and click Save to ensure the new record is successfully created. You can update the record again at any time.
- Make sure the learning objectives/description and key learnings & integration into practice are concise.
- Registrants can also upload a copy of the completed Learning Activity Notes form (available on our website at https://nlpb.ca/quality-assurance/professional-development/) as a supporting document and reference the information by entering “See attached” in the Learning Objectives/Description of Activity and Key Learnings & Integration into Practice fields.
Please note, when submitting documents digitally, please ensure your submissions adhere to the NLPB Scanning Guidelines and Checklist for Document Submissions Policy.
Professional development records from our previous registration system have been transferred over to the new Registrant Portal. If you have already recorded learning activities for the 2022 Professional Development (PD) Year, these records will be listed under Learning Activities for the current PD year in the new portal. However, the transferred records have not yet been fully formatted. Please be assured that the correct number of education credits and related documentation has been retained by NLPB. Registrants are not required to reformat this information; it will be reformatted at a later date.
| Documenting CEs
Service as a Preceptor PEBC Examination
For more information on documenting PEBC Examinations visit the PD FAQ – Special Situations on the NLPB website. For more information on documenting CEs, please review the Professional Development Requirements for Pharmacists and Pharmacy Technicians Interpretation Guide. |
Inviting Feedback on Strategic Direction
Reminder to registrants that NLPB is currently seeking feedback to prepare for a strategic planning session taking place at the end of November, in which the board of directors will re-evaluate NLPB’s current goals and objectives and provide direction for NLPB’s work over the next 3-5 years.
NLPB recognizes the vital importance of giving pharmacy professionals a voice in the development of a strategic plan that will guide the profession forward in the best interest of the public it serves. As such, we are asking you to help us identify ways that NLPB can promote access to safe and quality pharmacy care to support the public and the healthcare system that serves them.
We invite you to provide your feedback by answering a few brief questions, which can be accessed by clicking the link below.
https://www.surveymonkey.com/r/K7CPXJD
When providing feedback, please consider NLPB’s mandate for public protection, its vision and mission, and its core values of accountability, collaboration, integrity, respect, and transparency.
All feedback provided will be collected anonymously by our external strategic planning facilitator, Lynn Morrissey, who will compile the feedback and present it to NLPB. If you have any questions, you can contact Lynn at 709.687.2000 or lynnm@mun.ca.
The deadline for you to provide your feedback is November 3, 2022.
We look forward to receiving your feedback.
Student and Intern Administration of Injections
Due to recent inquiries, NLPB would like to provide clarification regarding the ability of pharmacy students and interns to administer injections. While pharmacy students and interns may not be independently authorized to administer injections, they are permitted to participate in this practice in the interests of learning, in accordance with NLPB policy, if the following conditions are met:
- The student or intern must be registered with the NLPB.
- The student or intern must have successfully completed an education and training program on the administration of injections that has received CCCEP accreditation or ALL of the education and training on the administration of injections provided as part of the pharmacy program curriculum.
- The student or intern must have current certification in CPR and First Aid from a recognized provider.
- The student or intern must be under the direct supervision of a pharmacist who has received authorization from the NLPB to administer drug therapy by inhalation or injection.
When providing direct supervision, the supervising pharmacist must be present when the activity is being performed and able to observe and promptly intervene to stop or change the actions of the individual being supervised. The supervising pharmacist is responsible and accountable for all components of the preparation and administration of the inhalants and injections, as well as ensuring the student or intern has met all NLPB requirements to administer inhalants and injections.
Additional Resources:
Administration of Drug Therapy by Inhalation or Injection Regulations
NLPB Standards of Practice – Administration of Drug Therapy by Inhalation or Injection
NLPB Policy – Pharmacy Students/Interns Administering Inhalations or Injections
Compounding Standards Deadlines – December 31, 2022
Registrants are reminded that the final implementation deadlines for the Compounding Standards are fast approaching – December 31, 2022. Pharmacists-in-charge must ensure that all the requirements of Phase 3 are met by that date, including:
- Non-Sterile Compounding
- Meeting all the requirements of Phases 1 and 2
- Completing the development of a quality assurance program
- Meeting all Level B and C requirements, as necessary
- Sterile Compounding
- Meeting all the requirements of Phases 1 and 2
- Completing the development of a quality assurance program
- Completing any necessary facility upgrades
Please see the Spring 2022 issue of The Apothecary for a detailed walk-through of the implementation of these two Standards of Practice.
Children’s Pain Reliever Shortage Update
Since earlier this year, supplies of various formats of non-prescription pediatric/infant and children’s acetaminophen and ibuprofen products have been limited in retail and pharmacy locations and hospitals across Canada. This shortage is due to unprecedented global demand, and even with ramped-up production, suppliers are unable to meet the needs.
The National Association of Pharmacy Regulatory Authorities (NAPRA), Health Canada, and manufacturers of these products have been in discussions on a biweekly basis over the duration of the shortage to discuss the situation and identify appropriate mitigation strategies.
If your pharmacy is experiencing a shortage of these medications, and you are approached about alternative options, pharmacists should use their clinical knowledge and professional judgment to assess each patient and advise on appropriate alternatives based on the individual circumstances. This may include recommending nonpharmacological options or advising on the appropriate use of chewable tablets or suppositories, or cutting or crushing a regular (adult) tablet. If you are asked to compound a solution or suspension, remember that you must only do so in accordance with the Standards for Pharmacy Compounding of Non-Sterile Preparations.
Health Canada has released the following statement and public advisory relating to the situation.
- Statement – Update on Supply of Children’s Acetaminophen and Ibuprofen Products
- Public advisory – Children’s ibuprofen/acetaminophen shortage: What you should know and do
NLPB’s Public Register
The Public Register is currently available by download only.
As NLPB moves to a new registrant portal, the current registrant portal from which the Public Register is generated is currently not accessible. As such, the register will be updated manually as registrant or licensee information changes. The date of the last update can be viewed above the links to each section of the register on the website. Should you have any questions or need further clarification regarding NLPB registrants and/or licensed pharmacies, please contact us at inforx@nlpb.ca.
Fall 2022 Issue of The Apothecary Published
Registrants are advised to view the latest issue of the NLPB Newsletter, The Apothecary, for updates on a number of issues, including:
- The approval of revisions to the Standards of Pharmacy Operation — Community Pharmacy, with a final implementation deadline of September 2023. Pharmacists-in-charge, pharmacists, and pharmacy technicians should review the standards in their entirety at their earliest convenience.
- The registrant audit plan and schedule for 2023.
- Tips and tricks for getting the most from a virtual community pharmacy practice site assessment.
Issue #88 – December 2022
Contacting NLPB
NLPB is currently receiving a high volume of emails and phone calls due to the upcoming annual renewal deadlines. As a result, the expected response time may be longer than usual. Please allow 3-5 business days (Monday-Friday, excluding holidays) for a response.
Should you be unable to speak directly to the appropriate staff member at the time of your call, please leave a detailed voicemail to ensure your inquiry or issue is addressed in a timely manner. We ask that you do not leave more than one message (email/voicemail), as it delays the staff’s ability to efficiently and effectively respond.
Children’s Pain Reliever Shortage Update
Since earlier this year, supplies of various formats of non-prescription pediatric/infant and children’s acetaminophen and ibuprofen products have been limited in retail and pharmacy locations and hospitals across Canada. This shortage is due to unprecedented global demand, and even with ramped-up production, suppliers are unable to meet the needs.
If your pharmacy is experiencing a shortage of these medications, and you are approached about alternative options, pharmacists should use their clinical knowledge and professional judgment to assess each patient and advise on appropriate alternatives based on the individual circumstances. This may include recommending nonpharmacological options or advising on the appropriate use of chewable tablets or suppositories, or cutting or crushing a regular (adult) tablet.
Canadian Pharmacists Association has released a useful resource for pharmacists to reference when counselling parents and caregivers on how to use 325 mg acetaminophen tablets to create children’s doses for ages 2-11.
The options listed above should be assessed before consideration of compounding the product; compounding should only be considered as a last resort.
Health Canada does not object to allowing compounding of these products without a prescription but within a patient-healthcare professional relationship until this shortage resolves. Health Canada recommends that, at the pharmacy level, appropriate documentation is maintained to demonstrate a patient-healthcare professional relationship.
If you are asked to compound a solution or suspension, remember that you must only do so in accordance with the Standards for Pharmacy Compounding of Non-Sterile Preparations. Pharmacy professionals are reminded:
- All compounding activities must be tied to a direct patient interaction, regardless of whether the product would be scheduled OTC. As such, it should be recorded in patient profiles. This step is particularly important to ensure the sale is traceable in the event of a “recall” or discovery of an error.
- Compounded products must be appropriately labeled when sold, including the strength of the medication.
- Pharmacists must take an active role in counselling patients or their agents on the strength of the compound and subsequent dose adjustments. The majority of recipes are for a different concentration than that which is commercially available. Patients must be aware that they can’t substitute 1:1 with their usual dose.
Role Distinction – Pharmacy Technician vs. Pharmacy Assistant
Registrants and pharmacists-in-charge are reminded that, in accordance with section 24.(2) of the Pharmacy Act, 2012, the designation, “pharmacy technician” is restricted to those registered as such with the NLPB. Unregulated pharmacy support staff may not be given or use this title but may be called “pharmacy assistants” or another similar title.
Registrants and pharmacists-in-charge are further reminded that, in accordance with section C.01.041.1 of the Food and Drug Regulations (Canada), only a pharmacist or a pharmacy technician may request a prescription transfer from another pharmacist or pharmacy technician pursuant to a request from a patient. Pharmacy assistants are not permitted to request or approve prescription transfers.
Compounding Standards Deadlines – December 31, 2022
Registrants are reminded that the final implementation deadlines for the Compounding Standards are fast approaching – December 31, 2022. Pharmacists-in-charge must ensure that all the requirements of Phase 3 are met by that date, including:
- Non-Sterile Compounding
- Meeting all the requirements of Phases 1 and 2
- Completing the development of a quality assurance program
- Meeting all Level B and C requirements, as necessary
- Sterile Compounding
- Meeting all the requirements of Phases 1 and 2
- Completing the development of a quality assurance program
- Completing any necessary facility upgrades
Please see the Spring 2022 issue of The Apothecary for a detailed walk-through of the implementation of these two Standards of Practice.
Logging in to the New Registrant Portal
Pharmacists and Pharmacy Technicians who have not yet logged into the new NLPB registrant portal and started recording their professional development activities are asked to do so now, in order to enable NLPB staff to assist in troubleshooting any issues you may encounter in a timely manner.
To access the portal please visit: https://nlpb.portalca.thentiacloud.net/webs/portal/service/#/login
Please use the most up-to-date version of one of the following recommended browsers: Chrome or Firefox.
All registrants should have received an email containing their login information and instructions for accessing the registrant portal on Thursday, September 22, 2022. Registrants are reminded that they must use the email address identified in the login information email to access the system. Please note, the email address is case-sensitive.
If logging into the system for the first time, click the Forgot Password button and enter your email address to have a new temporary password sent to you. Follow the instructions in the email to access the system. Do NOT use the Activate now link, as this will create a new account that will not be linked to your registration.
Please note, when logging in, the email address, password, and security questions are all case-sensitive. Should you enter the incorrect password or answer the security questions incorrectly more than 3 times, you will be locked out of the system. If you forget your password, please click the Forgot Password button prior to your third attempt and a new temporary password will be sent to your email address. Should you be locked out of your account, please contact registration@nlpb.ca to regain access.
Resources:
NLPB Registrant Portal User Guide – Renewing Registration
NLPB Registrant Portal User Guide – Adding Professional Development Activities
NLPB Registrant Portal User Guide – Adding/Updating Professional Liability Insurance
NLPB Registrant Portal User Guide – Adding/Updating Employment Information
| For more information on navigating the registrant portal, please visit our new NLPB Registrant & Pharmacy Portal web page. |
Recording Professional Development Activities in the Registrant Portal
Although the 2022 PD Year has ended, Pharmacists and Pharmacy Technicians can continue to document learning activities in the registrant portal. All required CEs for the 2022 PD Year must be documented in the online system prior to renewing your registration for 2023. However, documented activities can continue to be updated until the end of the professional development audit period in 2023.
To record your completed professional development activities in the registrant portal, follow the steps outlined in the NLPB Registrant Portal User Guide – Adding Professional Development Activities.
To assist registrants in adjusting to the 15-minute inactivity session timeout security feature, below are some tips for recording and successfully submitting learning activities.
- Save supporting documents in a dedicated location on your device so they are easy to find when you are ready to upload the files.
- Have your notes on hand when you are recording learning activities in the registrant portal.
- Make notes in a document on your computer so you can copy and paste the information into the online form in the registrant portal.
- When creating a new record, first upload the supporting document, enter the Program Title, insert placeholder text (i.e. TBD), and click Save to ensure the new record is successfully created. You can update the record again at any time.
- Make sure the learning objectives/description and key learnings & integration into practice are concise.
- Registrants can also upload a copy of the completed Learning Activity Notes form (available on our website at https://nlpb.ca/quality-assurance/professional-development/) as a supporting document and reference the information by entering “See attached” in the Learning Objectives/Description of Activity and Key Learnings & Integration into Practice fields.
Please note, when submitting documents digitally, please ensure your submissions adhere to the NLPB Scanning Guidelines and Checklist for Document Submissions Policy.
Professional development records from our previous registration system have been transferred over to the new Registrant Portal. If you have already recorded learning activities for the 2022 Professional Development (PD) Year, these records will be listed under Learning Activities for the current PD year in the new portal. However, the transferred records have not yet been fully formatted. Please be assured that the correct number of education credits and related documentation has been retained by NLPB. Registrants are not required to reformat this information; it will be reformatted at a later date.
| PREVIOUSLY DOCUMENTED LEARNING ACTIVITIES
Professional development records from our previous registration system have been transferred over to the new Registrant Portal. If you have already recorded learning activities for the 2022 Professional Development (PD) Year, these records will be listed under Learning Activities for the current PD year in the new portal. However, the transferred records have not yet been fully formatted. Please be assured that the correct number of education credits and related documentation has been retained by NLPB. Registrants are not required to reformat this information; it will be reformatted at a later date following the annual renewal period. |
| Documenting CEs
Service as a Preceptor PEBC Examination
For more information on documenting PEBC Examinations visit the PD FAQ – Special Situations on the NLPB website. For more information on documenting CEs, please review the Professional Development Requirements for Pharmacists and Pharmacy Technicians Interpretation Guide. |
Annual Registration Renewals
The deadline to document learning activities for the 2022 PD Year in the registrant portal and renew your registration for 2023 is Thursday, December 15, 2022.
As this will be the first renewal period using the new NLPB Registrant Portal, please refer to the NLPB Registrant Portal User Guide – Renewing Registration for step-by-step instructions for successfully renewing through the online system.
Please note, the deadline has now passed to complete learning activities for the 2022 PD Year. As per the NLPB Professional Development Requirements for Pharmacists and Pharmacy Technicians, Pharmacists and Pharmacy Technicians were required to complete a minimum of 15 CEUs between December 1 and November 30, of which half must have been accredited programs.
Annual Pharmacy Licence Renewals
Pharmacists in charge can access the Pharmacy Portal using the same login credentials as the Registrant Portal.
To access the portal please visit https://nlpb.portalca.thentiacloud.net/webs/portal/business/#/login.
Please use the most up-to-date version of one of the following recommended browsers: Chrome or Firefox.
Please note, the pharmacist-in-charge must first renew their registration before renewing the pharmacy licence.
Before renewing, pharmacists-in-charge should ensure they have a current list of all owners (including profession, mailing address, phone number, and email address) and a list of all registered and non-registered staff members (including pharmacy assistants and clerks). Prior to completing the licence renewal online, pharmacists-in-charge must ensure the staff listing and hours of operation are up-to-date and accurate in the pharmacy portal.
- For instructions on updating the Hours of Operation, please refer to the NLPB Pharmacy Portal User Guide – Adding/Updating Hours of Operation.
- For instructions on updating the Staff Listing, please refer to the NLPB Pharmacy Portal User Guide – Adding/Updating Staff Listing.
The pharmacist-in-charge is responsible for ensuring the licence has been renewed for 2023 and all regulated staff is registered to practice in 2023 before the end of the year.
Registration & Licensing Renewal Fees
Registration and Licensing is not complete until all fees are paid. Please refer to the 2023 Schedule of Fees for a list of applicable fees.
Additional Fees may apply:
- Any payment received after December 15, 2022, will be subject to an automatically applied 50% late fee.
- A processing fee will be charge for a declined credit card or an NSF cheque.
NLPB’s Public Register
The Public Register is currently available by download only.
As NLPB moves to a new registrant portal, the current registrant portal from which the Public Register is generated is currently not accessible. As such, the register will be updated manually as registrant or licensee information changes. The date of the last update can be viewed above the links to each section of the register on the website. Should you have any questions or need further clarification regarding NLPB registrants and/or licensed pharmacies, please contact us at inforx@nlpb.ca.
Regular reminders
- Registrant Contact Information – Registrants are responsible for ensuring that the contact information on their registrant profile, including email address and practice site, is accurate at all times. The NLPB primarily uses email communication to send newsletters, renewal reminders, practice site assessment information, professional development audit information, calls for interest for committees, and other alerts. If the email address on file is incorrect, important information may be missed and/or disclosed to the wrong person. If your contact information changes, please log into the NLPB Online Registrant Portal to update your file with your new contact information as soon as you can.
- Application Submission –NLPB receives many applications for processing. Unfortunately, some of the applications submitted are illegible, incomplete, or missing supporting documentation or payment, leading to delays in processing and approving the application. In order to ensure that your application is processed in a timely manner, please take a moment before submission to ensure that all fields are completed and legible and that all supporting documentation is included. These small steps will help improve the process for both the applicant and NLPB.
This e-newsletter contains information on a wide variety of topics intended to enhance the practice of pharmacy in the province of Newfoundland and Labrador. As it is published and circulated to all registrants on a monthly basis, it is the expectation of NLPB that all registrants are aware of the matters contained therein.