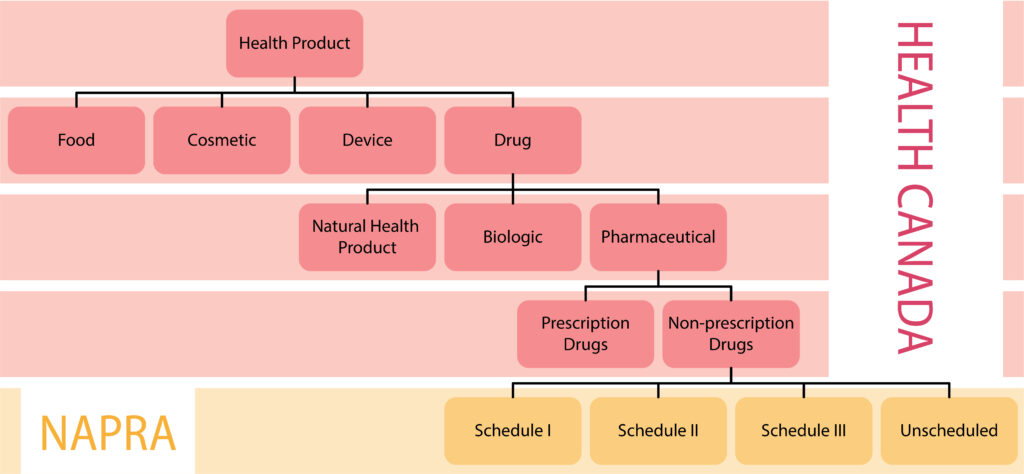
Health Canada and the National Association of Pharmacy Regulatory Authorities (NAPRA) both have roles related to drug scheduling in Canada. These roles are separate and distinct, with each organization performing specific functions within the drug scheduling process. The chart below outlines the classification system used by Health Canada and NAPRA.
Health Canada
Health Canada has the authority and responsibility to authorize health products for sale in Canada, including the responsibility to evaluate the safety, efficacy, and quality of health products. When reviewing health products, Health Canada will also classify the product into one of several product types and further classify drug products into additional categories. Drugs that Health Canada has determined require a prescription for sale in Canada are listed on the Health Canada Prescription Drug List or in the schedules to the Controlled Drugs and Substances Act and its regulations.
NAPRA
NAPRA’s role in the drug scheduling process occurs after Health Canada has authorized a drug for sale in Canada and determined whether or not the drug requires a prescription for sale. NAPRA only reviews products that have not been classified as requiring a prescription by Health Canada.
In 1995, the pharmacy regulatory authorities across Canada, endorsed a national drug scheduling model, to align the provincial/territorial drug schedules so that the conditions of sale for drugs would be more consistent across Canada. This harmonized national model is administered by NAPRA and is called the National Drug Schedules (NDS) program.
The NDS program consists of three schedules and four categories of drugs.
Provincial Implementation
NLPB has implemented a “schedule by reference” model, whereby the legislation references the NDS, and any scheduling decision made by NAPRA is automatically in force in Newfoundland and Labrador, other than in rare cases where provisions are implemented to account for needs specific to the province. These provisions can be referenced in the current drug schedule section below.
For more information, see the Newfoundland and Labrador Provincial Drug Schedules Policy.
PLEASE NOTE: In February 2024, the Minister of Health and Community Services amended the Pharmacy Regulations, 2014. This amendment effectively allows for naloxone hydrochloride, when being used for emergency use, to be unscheduled.
Current Drug Schedules
Updates
- February 24, 2023: Guidelines Regarding the Sale of Dimenhydrinate repealed
- National Drug Schedules (NDS) Updates
- July 25, 2024: National Drug Schedules: Final Recommendations for Desloratadine for Use in Children 2 to 11 Years
- June 11, 2024: Ephedrine and Pseudoephedrine removed from the National Drug Schedules as of June 10, 2024
- June 10, 2024: NDSAC Interim Recommendations on Desloratadine for Use in Children 2 to 11 Years
- February 8, 2024: Update to the NAPRA Natural Health Products Policy
- January 29, 2024: National Drug Schedules: Final Recommendations for Ophthalmic Brimonidine 0.025% and FDC of Acetaminophen and Ibuprofen
- April 25, 2023: National Drug Schedules: Final Recommendations for loratadine for use in children 2 to 11
- March 5, 2023: Next Meeting of the National Drug Scheduling Advisory Committee Scheduled for March 5, 2023